Biology Worthy of Life
An experiment in revivifying biology
The Unexpected Phases of Life
Stephen L. TalbottTags: form/molecular; gene regulation; holism/organism as a “formed stream”; machine idea/code; protein;
Following the discovery of the double helix in 1953 and the unraveling of
the
“genetic code”
in the early 1960s, molecular biologists began to get the feeling that the
main thing left was to fill in the details. Some even doubted whether any
fundamental discoveries remained to be made, and wondered whether their
own futures held any excitement. The ruling formula —
DNA
makes
RNA
RNA
makes protein
protein makes us
was understood to capture the essential workings of the organism, executed
at the molecular level with lock-and-key precision and code-like
necessity. If the governing principles were now elucidated, what was left
to motivate those who had previously savored the intoxicating race to lay
bare life’s most profound secrets?
Jump to the present and you find that the single emotive term occurring most frequently in the final paragraphs of molecular biological papers may well be the word “exciting”. This is more than biologists puffing their own work. The word is often justified. It’s as if we were traversing the exitements of that heady period after 1953, but this time without the extreme over-simplification: every few months we hear of unexpected and fundamental insights requiring us to reconceive an entire realm of old and once-mundane details. Researchers, it seems, can hardly help stumbling upon previously unimagined landscapes ripe for exploration. Reflect for a moment upon the last ten or fifteen years:
![]() The 98% of
DNA
once considered “useless” or “parasitic” is now being found to be
functionally decisive —
gene expression
depends upon it — and consequently receives at least as much attention
from geneticists as
genes
themselves.
The 98% of
DNA
once considered “useless” or “parasitic” is now being found to be
functionally decisive —
gene expression
depends upon it — and consequently receives at least as much attention
from geneticists as
genes
themselves.
![]() Likewise, the intricate, regulatory landscape of
chromatin,
which constitutes the structure of
chromosomes
and includes vastly more than
DNA,
may now draw as much attention from geneticists as
DNA
itself.
Chromatin
acts as a kind of
DNA
“chaperone” — a chaperone whose oversight and guidance is infinitely
nuanced, informed by the cell as a whole, and never fully predictable.
Likewise, the intricate, regulatory landscape of
chromatin,
which constitutes the structure of
chromosomes
and includes vastly more than
DNA,
may now draw as much attention from geneticists as
DNA
itself.
Chromatin
acts as a kind of
DNA
“chaperone” — a chaperone whose oversight and guidance is infinitely
nuanced, informed by the cell as a whole, and never fully predictable.
![]() Or we could look at the significant, but distinctively non-code-like
movements of
chromosomes
within the three-dimensional space of the
nucleus
— movements that are contextually sensitive and that teach us to see
gene expression
as something rather more like the outcome of a
nuclear
and even cellular dance than like a result of automatic
binding
by complementary molecules and lockstep processing by “molecular
machines”.
Or we could look at the significant, but distinctively non-code-like
movements of
chromosomes
within the three-dimensional space of the
nucleus
— movements that are contextually sensitive and that teach us to see
gene expression
as something rather more like the outcome of a
nuclear
and even cellular dance than like a result of automatic
binding
by complementary molecules and lockstep processing by “molecular
machines”.
![]() Then there is the fact that, while the search for fixed (crystallized)
structures of proteins,
DNA,
and molecular complexes remains a central preoccupation of molecular
biologists, the reality emerging from ever more sensitive imaging
techniques has more to do with structural plasticity and change
than with the static structure of rigid coding elements and meshing
machine parts. Everywhere the story is of dynamic, fluid form — an
interweaving of ongoing transformations whose ever-expressive choreography
constitutes the life of the cell.
Then there is the fact that, while the search for fixed (crystallized)
structures of proteins,
DNA,
and molecular complexes remains a central preoccupation of molecular
biologists, the reality emerging from ever more sensitive imaging
techniques has more to do with structural plasticity and change
than with the static structure of rigid coding elements and meshing
machine parts. Everywhere the story is of dynamic, fluid form — an
interweaving of ongoing transformations whose ever-expressive choreography
constitutes the life of the cell.
![]() And, very generally, there is the ongoing discovery of “crosstalk”,
linking almost every conceivable activity in the cell — activities that
researchers were once quite content to explore within the peaceful
isolation of their separate disciplines. The contemporary biologist can
no longer escape the unbounded complications — and enrichments — of
significant context.
And, very generally, there is the ongoing discovery of “crosstalk”,
linking almost every conceivable activity in the cell — activities that
researchers were once quite content to explore within the peaceful
isolation of their separate disciplines. The contemporary biologist can
no longer escape the unbounded complications — and enrichments — of
significant context.
Changes of state
Structure embodied in tangible or visible things has always been first to catch the biologist’s attention, even though life tells us that movement is primary and that things precipitate out of movement. In old age we may tend to ossify and crystallize, but the infant is wonderfully plastic, the embryo even more so, and early, post-zygotic development is virtually nothing but a “formed stream”. The point here is not that movement is lack of structure, but rather that it is an active structuring — a structuring that is more alive than its frozen end products. Even our bones at middle age are still being re-shaped by the patterns of our movement.Plastic structure confronts us everywhere in cytoplasm (the interior content of cells). Cytoplasm contains various membrane-bound organelles such as the nucleus and mitochondria, as well as a finely filamentous, highly dynamic cytoskeleton that helps give overall adaptive shape and mechanical support to the cell while also facilitating cell migration and the internal movement of substances. But beyond these structures, there are more subtle expressions of form that biologists only now have the technical tools to begin penetrating.
It’s become increasingly clear in recent years, that, quite apart from its organelles and cytoskeleton, the cytoplasm is elaborately and “invisibly” organized. Various macromolecular complexes, in more or less defined mixes, congregate in specific locations and sustain a collective identity, despite being unbounded by any sort of membrane. Here we’re looking at structure without even a pretense of mechanically rigid form. How do cells manage that?
A couple of years ago a research duo from the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden, and the Department of Chemical and Biological Engineering at Princeton University, writing for the journal, Developmental Cell, framed the problem this way:
“Non-membrane-bound macromolecular assemblies found throughout the cytoplasm and nucleoplasm [contents of the nucleus] … consist of large numbers of interacting macromolecular complexes and act as reaction centers or storage compartments. ... We have little idea how these compartments are organized. What are the rules that ensure that defined sets of proteins cluster in the same place in the cytoplasm? (Hyman and Brangwynne 2011*)
Even more puzzling, a “compartment” can maintain its identity despite the rapid exchange of its contents with the surrounding cytoplasm. “Fast turnover rates of complexes in compartments can be found throughout the cell. How do these remain as coherent structures of defined size and shape when their components completely turn over so quickly?”
Part of the picture now coming into focus has to do with the phases of matter and the transitions between these phases. (Think, for example, of the solid, liquid, and gaseous phases of water, or of solutions and gels — matter in different states.) For example, it’s possible for well-defined droplets of one kind of liquid to occur within a different liquid, or within a gel, just as bits of ice and air bubbles can occur in water.
Hyman and Brangwynne pointed in particular to studies suggesting that complexes of RNA and protein — called ribonucleoproteins or RNPs — often amass in liquid form as droplets within the cytoplasm. That is, they are not dissolved in the cytoplasm, but form distinctive RNA-protein liquids that separate out as droplets within the larger cytoplasmic medium. The overall concentration of RNAs and proteins in the droplets may not differ much from the surrounding cytoplasm, but the concentration of particular molecules may be much greater in the droplets, conferring specific and efficient functions upon the assemblies. Enzymes and reactants can rapidly diffuse within the liquid droplet, while also moving with relative ease across the boundary between droplet and cytoplasm. Yet this boundary can remain until phase-changing environmental conditions occur — conditions that might involve temperature, pH, salt concentration, electrical charge, and so on. A very subtle change — originating, say, from an extracellular signal — can yield a dramatic transformation of cytoplasmic organization, just as a slight change in the temperature or salinity of water can shift an ice-forming condition to an ice-melting one.
“The physical picture that emerges from these studies”, according to Hyman and Brangwynne, “is of a cytoplasm … that represents complex emulsions of dynamic liquid-phase droplets of RNA and protein”.
Included in this picture are numerous more short-lived RNA-protein assemblies dedicated to one-time tasks, such as the repair of a double-strand break in DNA. The necessary molecules assemble, perform their work, and then disperse. “It is tempting to speculate that condensation of liquid-like protein phases could be a general conceptual framework for understanding the formation of such compartments”, nucleated around specific sites such as double-strand breaks.
But there is no reason to speak only of liquid-phase RNP droplets.
Phase transitions in egg, embryo, and protein
A very recent paper from a University of Colorado group provides concrete evidence for the importance of various phases and the transitions between them. They looked at large-scale assemblies of RNPs that form liquid, semi-liquid, or solid aggregates within both cytoplasm and nucleoplasm, and noted how regulated phase transitions among these states play important roles in life processes:Living cells organize functions not only by membrane compartmentalization, but also by assembling supramolecular structures within aqueous environments. ... Supramolecular assemblies are emerging as a prominent feature of gene expression pathways. ... Specific RNPs often coassemble into a remarkable diversity of large RNP granules or domains. In the nucleus, these structures include the nucleolus, Cajal bodies, and a variety of other nuclear RNP particles. Diverse RNP assemblies are also common in the cytoplasm … Like chromatin, these RNP assemblies are regulated by developmental programs and cell state changes, suggesting important roles in controlling cell fates. (Hubstenberger, Noble, Cameron and Evans 2013*).
Hubstenberger et al. looked at early developmental processes in the nematode, Caenorhabditis elegans. They show that regulated interactions drive RNP phase transitions in the cytoplasm of immature C. elegans egg cells, conducing to reorganization of RNP assemblies as they shift among solid, semi-liquid, liquid, and diffuse states. And so, for example, when immature egg cells are stimulated toward maturity, many highly viscous, semi-liquid RNP assemblies become more dynamic liquid droplets and their constituents become more mobile, which is thought to promote rapid changes in RNP organization after fertilization — changes, for example, that facilitate the differentiation of cells in the growing embryo.
Such RNP phase transitions, according to the researchers, “are controlled with surprising precision in early development, leading to starkly different supramolecular states that alter RNP organization and dynamics. ... Pathways of mRNA regulation control these transitions. ... Reversible interactions among thousands of RNP complexes impart regulated patterns of RNP dynamics, and large-scale organization of gene expression pathways in the cytoplasm”.
Phase transitions seem to be receiving rapidly increasing attention, although the many reports tend to come from disconnected subspecialties and have yet to result in a visible “movement” within molecular biology and cell physiology. But the time for a broader awareness is evidently drawing near. Peter Tompa, a structural biologist from Vrije Universiteit Brussel in Belgium, remarks in a current paper that sol-gel transitions1 “appear to be critical in an ever increasing number of biological systems and emerge as a novel physical regulatory and organizational principle of cell physiology”.
Tompa himself urges the importance of phase transitions involving hydrogels2 constituted from intrinsically disordered proteins in the cytoplasm. Noting the importance of compartmentalization within eukaryotic cells, he suggests that random encounters between freely diffusing molecules cannot account for the kinds of biological interactions we observe. Therefore,
the operation of the cell must be dominated by a hierarchic assembly process driven by assembly pathways and dedicated transport mechanisms. The gel phases described might regulate the movement of regulatory proteins in and out of organized subcellular domains, and impart transitions between distinct states. (Tompa 2013*)
As an aside: you see in this passage the rather strange lacuna characteristic of virtually all molecular biological writing today. A “regulatory” action is invoked as if it were explanatory, without notice being taken of the fact that it only raises wider questions about regulation. In the present case, gel phases regulate the movement of proteins that are themselves regulatory and in fact are part of the means by which the “regulating” gel is formed or dissolved. There’s no clear cause-and-effect relationship here, and we can’t say in any definitive sense who is regulating whom, despite a naïve use of language that suggests we can.
Moreover, the obvious next question — How are gel phases themselves regulated so as to perform their coordinating role? — is not even alluded to. It’s not that one has to address everything when talking about just one thing. The point, rather, is that biologists today are continually giving the impression with their language that one thing explains another, when the truth of the matter is that they are (admirably) filling out a picture no single aspect of which ever fully explains other aspects. This is because of the integral sort of unity we witness in the organism. Partial activities participate in an ordered whole and do their part in helping us to see that whole, but no partial activity explains the whole that it subserves.
I’ll have much more to say about this in the future. In any case, the foregoing barely touches upon the diverse but scattered literature dealing with phases and phase transitions in the cell. But it has, I hope, given a taste of things to come.
And then there is the water itself
I have long thought that some day water will be seen as the most fundamentally important, “information-rich” biomolecule of all and that revelations in this regard will outweigh in significance even those concerning the structure of the double helix. No biologist today would suggest such a thing — and I am not defending the idea here, if only for lack of ability. I am content to let time decide the matter. But I was particularly pleased to find while writing this article that Nature columnist Philip Ball once entitled a piece, “Water as a Biomolecule”. In it he wrote:Water is not simply ‘life’s solvent’, but rather an active matrix that engages and interacts with biomolecules in complex, subtle and essential ways. ... Water needs to be regarded as a protean, fuzzily delineated biomolecule in its own right. (Ball 2008a*; see also Ball 2008b*)
In a more recent paper, Ball (2011*) summarized some work bearing on the role of water in biological contexts. The main topic had to do with the relation between water, the binding cavity of an enzyme, and the substrates to which the enzyme binds. It turns out, according to the authors of a study Ball cites, that “the shape of the water in the binding cavity may be as important as the shape of the cavity”. Ball goes on to remark:
Although all this makes for a far more complicated picture of biomolecular binding than the classic geometrical “lock and key” model, it is still predicated on a static or quasi-equilibrium picture. That, too, is incomplete ...
Then he cites another paper on enzyme-substrate binding. There it is revealed that, before the binding is complete, water movement near the enzyme is retarded. “Crudely put, it is as if the water ‘thickens’ towards a more glassy form, which in turn calms the fluctuations of the substrate so that it can become locked securely in place. It is not yet clear what causes this solvent slowdown as a precursor to binding; indeed, the whole question of cause and effect is complicated by the close coupling of protein and water motion and will be tricky to disentangle. In any event, molecular recognition here is much more than a case of complementarity between receptor and substrate — it also crucially involves the solvent”.
All this, finally, “suggests that changes in protein and solvent dynamics are not mere epiphenomena, but have a vital role in substrate binding and recognition”.
A concluding note
Structural biologists Mark Gerstein and Michael Levitt (the latter a 2013 Nobel laureate in chemistry) wrote an article in Scientific American entitled “Simulating Water and the Molecules of Life”. In it they mention how early efforts to develop a computer simulation of a DNA molecule failed; the molecule almost immediately broke up. But when they included water molecules in the simulation, it proved successful. “Subsequent simulations of DNA in water have revealed that water molecules are able to interact with nearly every part of DNA’s double helix, including the base pairs that constitute the genetic code”.
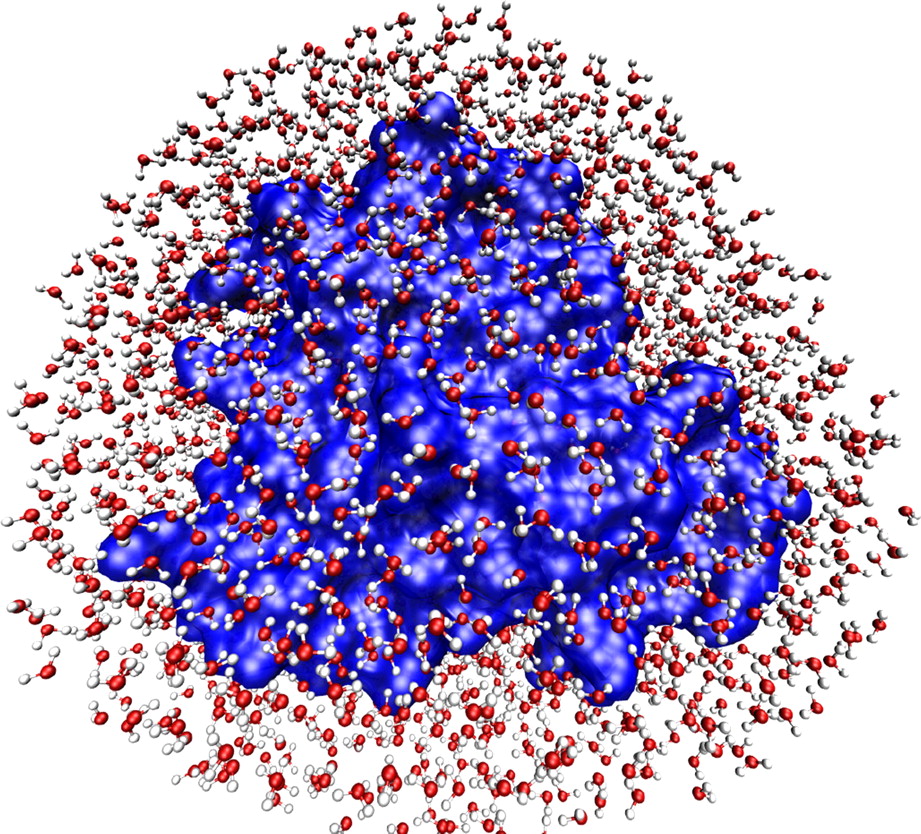 At top, a cartoon-schematic image of a protein, showing certain basic
structural elements. At bottom, an image of a (different) protein’s
hydration shell, where the small, red-and-white molecules are water
molecules. Of course, both images represent almost nothing of the
reality of the molecules (whatever we might take that reality to
be), but only certain abstractly conceived features.
[Image at top
© Richard Wheeler (GNU FDL).
Image at bottom from
H. Frauenfelder et al.,
PNAS vol. 106, no. 13 (2009), pp. 5129-34.
At top, a cartoon-schematic image of a protein, showing certain basic
structural elements. At bottom, an image of a (different) protein’s
hydration shell, where the small, red-and-white molecules are water
molecules. Of course, both images represent almost nothing of the
reality of the molecules (whatever we might take that reality to
be), but only certain abstractly conceived features.
[Image at top
© Richard Wheeler (GNU FDL).
Image at bottom from
H. Frauenfelder et al.,
PNAS vol. 106, no. 13 (2009), pp. 5129-34.
Early attempts to simulate protein molecules produced an analogous difficulty, with the same, water-dependent resolution. Gerstein and Levitt concluded their article with this remark:
When scientists publish models of biological molecules in journals, they usually draw their models in bright colors and place them against a plain, black background. We now know that the background in which these molecules exist — water — is just as important as they are.
That was in 1998. Fifteen years later the background remains largely blank, even if we are now seeing signs of change. Philip Ball (who likes to cite that Gerstein/Levitt remark, and who reproduces two images like those to the right), has recently noted “an interesting sociological question”, namely, “why certain communities in science decide that particular aspects of a problem are worth devoting a great deal of attention to while others become minority concerns, if not in fact regarded as somewhat suspect and disreputable”. He adds:
Why should we place so much emphasis, for example, on determining crystal structures of proteins and relatively little on a deep understanding of the [water-related] forces ... that hold that structure together and that enable it to change and flex so that the molecule can do its job? (Ball 2013*)
Certain peculiar historical episodes have contributed to the disreputability of water as a “molecule of life”. But surely part of the answer to Ball’s question has to do with the longstanding distortion of biology due to the emphasis upon code and mechanism. It is much easier to imagine the step-by-step execution of a computer-like code or the clean insertion of a key into a lock than it is to come to terms with fluid transformations — that is, with what is actually life-like.
As with so many aspects of the more dynamic biology emerging today, the importance of phase transitions was foreseen by prominent biologists during the first half of the twentieth century. For example, E. B. Wilson and others considered the cell cytoplasm to be a kind of emulsion, packed with liquid protein “coacervates”. But, as Hyman and Brangwynne, who mention this fact, also point out, such ideas about distinct cytoplasmic states “dropped out of favor with the molecular biology revolution, with its focus on crystals and stereospecific [think lock and key] interactions between proteins”.
The high era of molecular biology that followed upon discovery of “the”
structure of the
double helix,
was indeed the Age of Simplicity. We can be thankful that the feverish
enchantment of code and crystal is now showing signs of passing. And we
can be absolutely sure that the swelling tide of discovery in molecular
biology will never again allow us to be tempted by simplistic formulas
such as
DNA
makes
RNA
RNA
makes protein
protein makes us
Notes
1. A sol-gel transition occurs between a solution state, where one substance is dissolved in another, and a gel state. The latter consists of a solid molecular lattice that is expanded throughout its volume by a fluid — water, in the case of a hydrogel. The fluid may constitute over 99% of the volume of the gel, yet the solid lattice prevents the gel from flowing like a liquid.
2. See previous note for the meaning of “hydrogel”.
Tags: form/molecular; gene regulation; holism/organism as a “formed stream”; machine idea/code; protein;
Sources: Ball, Philip (2008a). “Water as a Biomolecule”, ChemPhysChem vol. 9, pp. 2677-85. doi:10.1002/cphc.200800515
Ball, Philip (2008b). “Water as an Active Constituent in Cell Biology”, Chemical Reviews vol. 108, no. 1, pp. 74-108. doi:10.1021/cr068037a
Ball, Philip (2011). “More Than a Bystander”, Nature vol. 478 (Oct. 27), pp. 467-8. doi:10.1038/478467a
Ball, Philip (2013). “Concluding Remarks: Cum Grano Salis”, Faraday Discussions vol. 160, pp. 405–14. doi:10.1039/c2fd20126g
Hubstenberger, Arnaud, Scott L. Noble, Cristiana Cameron and Thomas C. Evans (2013). “Translation Repressors, an RNA Helicase, And Developmental Cues Control RNP Phase Transitions during Early Development”, Developmental Cell vol. 27 (Oct. 28), pp. 161-73. doi:10.1016/j.devcel.2013.09.024
Hyman, Anthony A. and Clifford P. Brangwynne (2011a). “Beyond Stereospecificity: Liquids and Mesoscale Organization of Cytoplasm”, Developmental Cell vol. 21 (July 19), pp. 14-6. doi:10.1016/j.devcel.2011.06.013
Tompa, Peter (2013a). “Hydrogel Formation by Multivalent IDPs: A Reincarnation of the Microtrabecular Lattice?”, Intrinsically Disordered Proteins 1:e24068 (Jan./Feb./Mar.). doi:10.4161/idp.24068
Further information: See in particular Getting Over the Code Delusion: Biology’s Awakening. For a brief discussion of disordered proteins, see “Are Disordered Proteins Really Disordered?”. An example of early twentieth-century biological thinking that appears extremely forward-looking even in our own day can be found in this excerpt from marine biologist E. S. Russell’s book, The Interpretation of Development and Heredity. On the tension between plasticity and relatively fixed form in the organism, see Free Life and Confining Form.
This document: https://bwo.life/org/comm/ar/2013/phases-of-life_13.htm
Steve Talbott :: The Unexpected Phases of Life

